Freeze excursions are invisible, but they don’t have to be undetectable, here’s how to prove your cold chain held when it matters most.
The invisible failure
A specialty pharmacy ships a large shipment of biologics this week. Validated packaging. Temperature-controlled shipping. Trusted carrier.
On Tuesday, 12 vials freeze in a delayed truck outside Chicago. The packaging looks fine. The courier scans “delivered.” The pharmacy dispenses the medication.
No one knows the product is corrupted.
Freeze damage doesn’t leave visible traces. Biologics, insulin, vaccines, and GLP-1s lose potency permanently when frozen. And they’re happening most often in the places you can’t see: cold chain routes, cross-dock transfers, and direct-to-patient doorsteps.
The gap isn’t in your packaging qualification. It’s in knowing what actually happened between your dock and final delivery.
The cold chain’s blind spots
We see this misconception constantly: “Our packaging passed IQ/OQ/PQ, so we’re covered.” Validated packaging tells you what should happen under test conditions. It doesn’t tell you what did happen when:
- A courier leaves a package on a front porch during a polar vortex
- Cross-dock delays expose shipments to sub-zero loading docks for hours
- Gel packs over-perform in winter and freeze products rated for 2–8°C
- Delivery vehicles lose temperature control overnight in cold climates
The products at highest risk
Biologics and protein-based drugs irreversibly lose efficacy when frozen. Biologics, insulin. GLP-1s, vaccines, tissue samples, donor milk and diagnostic specimens are all at risk.
These aren’t products you can inspect visually. A frozen-then-thawed vial looks identical to a properly stored one. But the therapeutic effect is gone.
The cost
Product replacement: $500 to $3,000 per specialty biologic dose. Patient safety incidents. Therapy delays. Regulatory scrutiny. And the compliance risk of shipping product without confirming temperature maintenance.
The question QA teams ask us: How do we prove the cold chain held when the product traveled outside our direct control?
Visual confirmation for every shipment
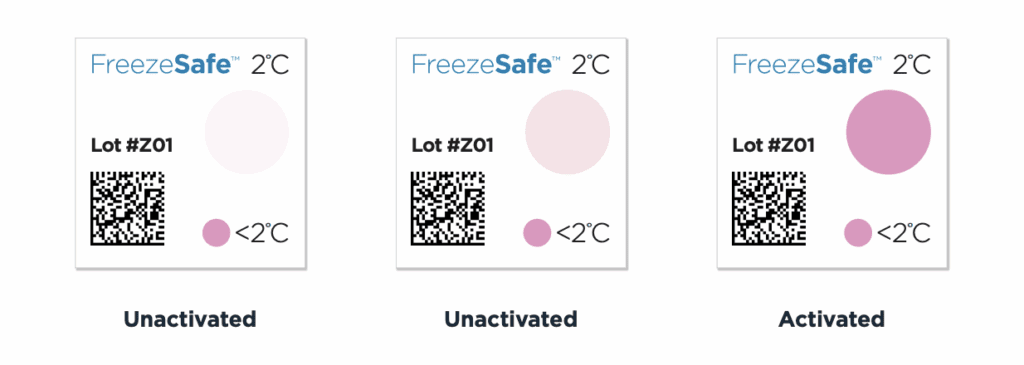
FreezeSafe sensors turn from clear to magenta when temperatures drop below validated thresholds. The color change is irreversible and requires no interpretation.
How it works
- -3°C: For products with extreme cold tolerance
- 0°C: Standard freeze protection for most biologics
- 2°C: For temperature-sensitive cold chain products rated 2–8°C
- 5°C: For products at the upper end of refrigerated ranges
Each threshold includes a time delay to filter brief exposure:
- 0°C threshold: 60 minutes
- 2°C threshold: 90 minutes
- -3°C and 5°C thresholds: 120 minutes
This prevents false positives from momentary door-open events while capturing sustained freeze conditions that damage product.
Form factor: 21 × 21 mm with an adhesive-covered back. Fits on vials, cartons, insulated shippers, or outer packaging. No batteries. No activation steps. No calibration.
Deploy on every unit
Unlike data loggers that can cost $50+ per device and require retrieval, FreezeSafe indicators cost pennies per unit. This makes unit-level protection practical for high-volume specialty pharmacy, diagnostics, and direct-to-patient distribution.
Receiving staff see the indicator. Clear means accept. Magenta means reject. No waiting for devices to download. No ambiguity.
Why this matters
Root cause analysis: Identify patterns across carriers, lanes, or seasonal conditions.
Regulatory documentation: Generate audit trails that support electronic record requirements for quality systems and FDA inspections.
Continuous improvement: Quantify freeze risk by route, packaging type, or carrier performance.
Specialty pharmacies shipping thousands of units weekly can’t retrieve data loggers, but FreezeSafe makes it possible to verify product integrity instantly at receiving.
Complementing packaging qualification with real-world evidence
Here’s the counterargument we hear: “If our packaging is validated, why do we need indicators?”
Packaging validation (IQ/OQ/PQ) qualifies thermal performance under controlled test conditions. But validation doesn’t account for:
- Carrier delays that extend transit time beyond the qualified window
- Improper handling that compromises packaging integrity
- Extreme weather beyond your test parameters
- Multi-party handoffs where accountability blurs
FreezeSafe fills the gap

Indicators provide in-transit confirmation that your packaging performed as designed or flag when it didn’t. Even with “perfect” packaging validation, real-world scenarios can introduce risks that a packaging study can’t predict. For example, a validation study may not account for unexpected weekend delays that leave a direct-to-patient shipment on an unheated porch through a freezing night. In these situations that fall outside of lab-tested parameters, the indicators provide the crucial evidence that a packaging study couldn’t foresee.
Use cases
Cold chain validation: Deploy indicators across high-risk routes to confirm packaging performance in real-world cold weather.
Direct-to-patient: Track freeze risk in ship-to-home workflows where products sit on doorsteps or in unheated mailrooms.
High-value shipments: Protect every dose of biologics worth $1,000+ per unit with serialized, traceable freeze evidence.
Sensors support regulatory inspections and payer audits by demonstrating proactive temperature monitoring and documented accept/reject criteria.
Practical steps for specialty pharmacy, diagnostics, and last-mile logistics
Step 1: Select thresholds
Match FreezeSafe thresholds to your product’s labeled storage range and stability data.
Step 2: Choose deployment strategy
- Unit-level: Apply indicators to every vial, kit, or package for maximum visibility
- Shipment-level: Apply to inner packaging or insulated shippers for aggregated ambient temperature monitoring
- Risk-based: Prioritize high-value products, cold chain validation, or direct-to-patient shipments
Step 3: Integrate into workflows
Receiving staff check indicators as part of standard acceptance procedures. The readings give quality and operations teams immediate confirmation of product integrity.
Step 4: Trial and refine
Request units for validation across your highest-risk distribution lanes. Start with your most vulnerable routes: cold chain routes, cross-dock hubs, or ship-to-home workflows, and scale based on findings.
Cost, compliance, and patient safety
Prevent losses
One prevented freeze excursion on a specialty biologic shipment ($1,200+ per dose) justifies hundreds of indicator deployments.
Simplify compliance
Audit-ready documentation supports FDA inspections and internal quality systems without additional infrastructure.
Protect patients
Freeze-damaged product that reaches patients risks therapy failure, adverse reactions, and safety incidents. Indicators stop bad product before it’s dispensed.
Scale without complexity
No calibration. No device retrieval. No battery management. FreezeSafe scales to high-volume operations without operational overhead.
Cold chains don’t wait
Freeze excursions can be invisible. But they don’t have to be undetectable.
FreezeSafe indicators provide immediate, irreversible, tamper-proof evidence of freeze exposure.
From validated packaging studies to last-mile patient delivery, you need freeze protection you can prove.
—–
You might also like:







