Freeze Indicator Case Study
Product Solution: FreezeSafe Indicator
Read The Case Study
Freeze Indicator Case Study
Company Profile
Application: Cold chain temperature monitoring
Challenge: The security of knowing if any products have frozen during transport

Unactivated

Unactivated

Activated
Innovative Protection: Freeze Detection for Pharmaceutical Products Boosts Safety Standards
Maintaining the right temperature in the cold chain is an absolute must!
Ensuring the temperature is constantly monitored and adjusted to the correct level is critical. Without this, the quality of your products is compromised, leading to health hazards and financial losses.
The SpotSee FreezeSafe indicator provides irreversible evidence if your product has experienced unacceptably low temperatures, ensuring the safety and quality of pharmaceutical products in the cold chain.
While some pharmaceutical products require freezing to avoid bacterial contamination, harmful reactions, instability, and degradation, it is detrimental for others. Pharmacies, caregivers, and patients need to know whether their products freeze in transit, and SpotSee’s freeze indicator provides that information.
Despite some vaccines needing frozen or deep-frozen temperatures, many vaccines, biologics, and other medications are ruined if frozen. HUMIRA® (adalimumab), a treatment for ulcerative colitis, and Pradaxa (dabigatran), an anticoagulant pill, are two of many possible examples.
More recent weight loss drugs, like Wegovy, are altered and rendered ineffective by freezing. Some medications, like insulin (used to treat the approximately 29 million Americans with diabetes), become completely inactive when frozen, which can lead to serious complications or even death.
Drug developers and distributors go to great lengths to use the proper protective packaging when shipping therapeutics and diagnostics, but sometimes those materials fail, are damaged, or aren’t used properly.
One large pharmaceutical logistics provider detailed such an event at a conference a few years ago:
A warehouseman was hypervigilant about maintaining refrigerated temperatures for a particular cold-chain package, so he placed it in the refrigerator until the carrier arrived. That drop in temperature was enough to cause the contents to freeze, ruining the therapeutic.
Clearly, accidents happen.
With telemedicine, more medications and diagnostics are shipped directly to patients’ homes and from the home to a lab. Before the Covid-19 pandemic, the ship-to-home medication market was already expanding, and that growth has continued.
This means therapeutics, assays, and patient samples (such as blood or other fluids) may be exposed to cold temperatures as they move the last mile or around the country, sitting in a cargo hold, on porches, or in mailboxes.
Infusion pharmacies and clinics, distributors, hospitals, physicians’ offices, and retail pharmacies must ensure no accidents affect their products, whether incoming or outgoing. They and their patients must be assured that products are safe from freezing temperatures throughout the supply chain, thus ensuring that those therapeutics, vaccines, or diagnostics are as beneficial now as when they were manufactured.
Unfortunately, freezing isn’t always immediately evident, and drugs may freeze and thaw during shipping without anyone knowing. While some become cloudy, others have no visible signs of freezing. Consequently, not even pharmacists can look at a drug and know whether it froze and thawed before they received it.
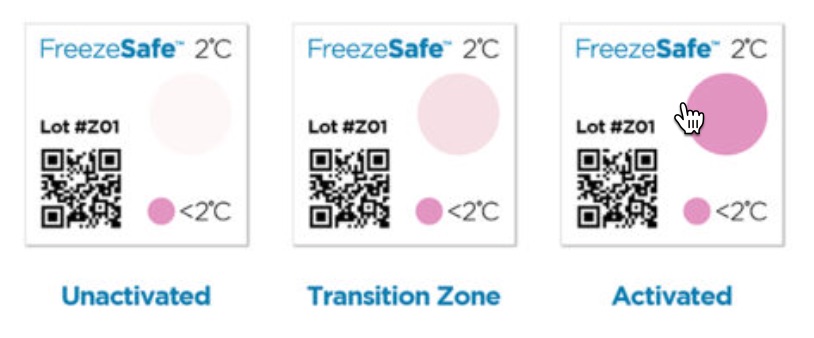
That is why SpotSee created the FreezeSafe product line. FreezeSafe single-use indicators clearly show whether unacceptably low temperatures have occurred between when the indicator was affixed to the product and when it arrived.
They provide a simple visual check that immediately alerts the recipient if the temperature is unacceptable.
- When the irreversible indicators change color from clear to magenta, patients, pharmacists, or others can see whether temperatures have dropped to dangerously low levels.
- To trigger the irreversible color change, the temperature must fall below 32°F/0°C within 60 minutes, below 36°F/2°C within 90 Minutes, or below 27°F and 41°F /-3°C and 5°C within in 120 minutes
- Each unit is serialized for traceability, proving the correct temperature has been maintained.
- The indicator easily adheres to irregularly shaped or small objects.
These freeze indicators were designed in a format that fits easily on life science products, while still including the information important to SpotSee’s customers. And, because they are flat, manufacturers can continue to use their usual packaging materials.
This low-cost freeze indicator is self-adhesive, so users just peel off the protective backing and stick the indicator onto the product or into the shipment.
SpotSee’s FreezeSafe are individual indicators, also found in the SpotSee ColdChain Complete and ColdChain Complet XS, which show when the temperature is too hot or too cold.
They provide an easier, worry-free, quick-reading, reliable, and smarter way to ensure products are kept at the right temperature.

Unactivated

Transition Zone